Collaboration Agreements with Swiss university hospitals
SPHN supports the five Swiss university hospitals in establishing the core infrastructure to make clinical routine data FAIR* for research at the national level.
Since 2018, SPHN has been engaging with the university hospitals in Basel, Bern, Geneva, Lausanne, and Zurich through collaboration agreements (CA). The focus of these collaborations lies on establishing a joint regulatory framework for accessing and using clinical routine data for research, based on the concept of a network of trusted partners. Technically, clinical data platforms within university hospitals facilitate data recruitment and enable data interoperability at the national level. Some examples of current deliverables for university hospitals are listed on the bottom of this page.
*The FAIR principles require that data are Findable, Accessible, Interoperable, and Reusable. Sensitive health data cannot be openly accessible to third parties, but taking regulatory requirements into account, the data should be FAIR – also with reproducibility and sustainability in mind. The main goal of the FAIR principles is the optimal preparation of research data for reuse by humans and machines.
Where can researchers inquire about data from the university hospital data platforms?
Contact information for inquiries, support and requests from clinical data platforms
Each university hospital maintains a single-point-of-contact for inquiries, support and requests concerning data from its clinical data platform:
Contact University Hospital of Basel (USB)
Contact University Hospital of Bern (Insel)
Contact Lausanne University Hospital (CHUV)
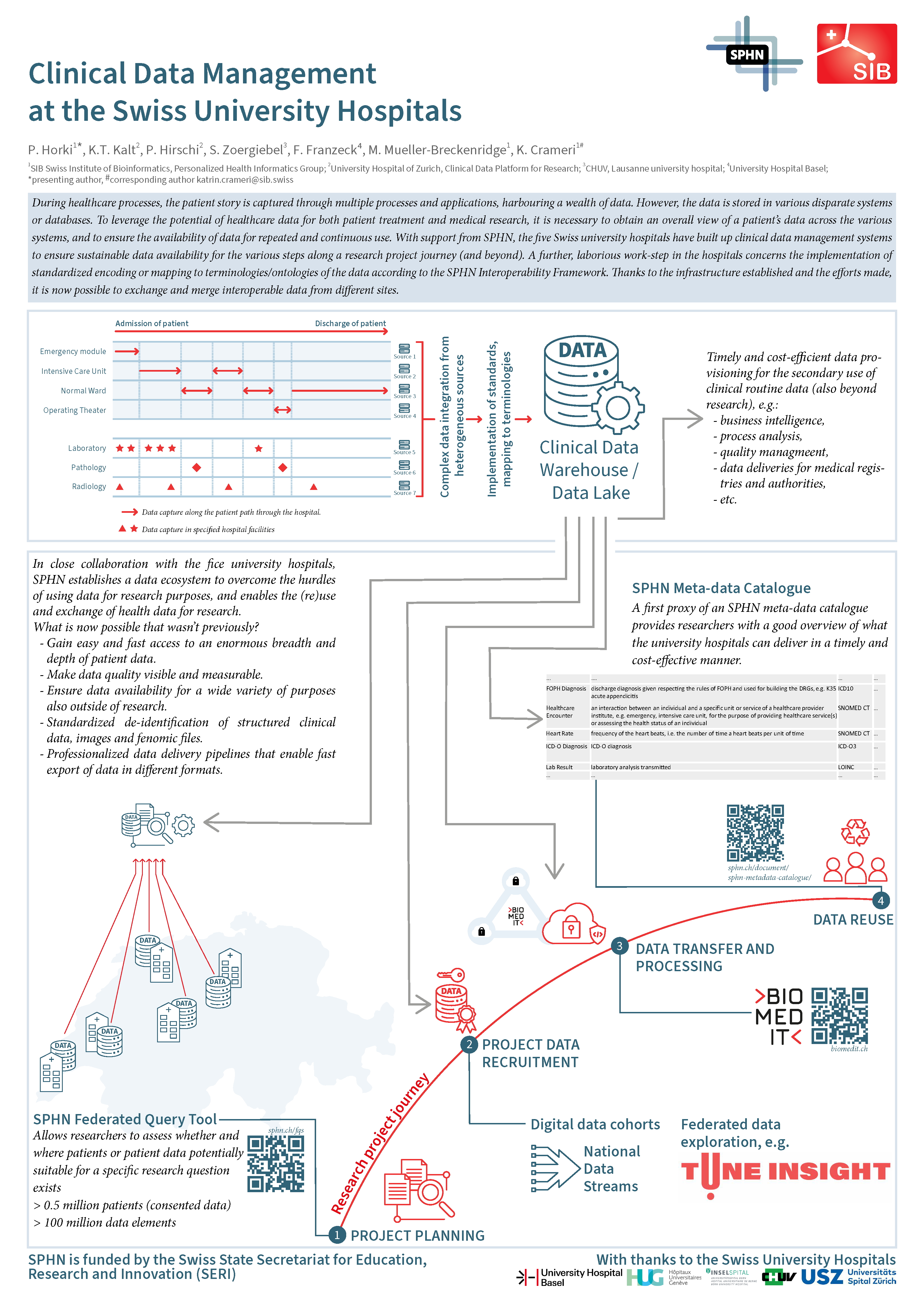
What data are available from university hospitals?
SPHN Federated Query System
The Federated Query System (FQS) provides researchers with information about basic clinical data (with General Consent) present on the data platforms of the five university hospitals. With the FQS, researchers can assess the feasibility of a clinical research question by querying anonymized and nationally harmonized data coded in national and international terminologies to assess whether patients or patient data exist at one or several of the Swiss university hospitals (UHs). Further information about available data or requests for access to data need to be addressed to the respective single-point(s)-of-contact.
SPHN Metadata Catalogue
Data that were provided in the past from the clinical data platform to a research project can be more efficiently mobilized than data that need to be identified, extracted and mapped from scratch. As a first approximation to an SPHN metadata catalogue foreseen in the future, a table provides information about the datasets and data elements from selected SPHN projects.
Further information about the clinical data management in the university hospitals can be found on the poster presented by Petar Horki (PHI group, Basel) at the second Personalized Health Day 2022 (see below)
Examples of deliverables of the Collaboration Agreements 2021-2024 between SPHN and the university hospitals
A roadmap specifies jointly agreed deliverables that are annually updated and mutually approved.
List of deliverables (year 3) to be added
Year 2 (01.07.2022 – 30.06.2023)
Data exchange framework
- UH maintains a transparent process with objective criteria for sharing clinical routine data for research with partners outside of the UH.
- UH maintains a single-point-of-contact for all data requests by SPHN-funded projects.
- UH formally answers requests for signing of a DTUA (according to SPHN template) to the single-point-of-contact within 30 working days (ideally, including signing of DTUAs and MTAs). For denied request, the reasons for rejecting the request will be provided in writing.
- UH commits to support the use and further development of the SPHN Data Transfer and Use Agreement (DTUA).
Data provision:
- UH provides a quote for data extraction and data delivery according to the SPHN Interoperability Framework to projects and National Data Streams (NDS) applying for SPHN funding and being preselected by SPHN bodies. Quotes shall address how change requests by projects are handled.
- According to projects’ milestones: UH provides extracted data in SPHN-defined format (if available) to SPHN-funded projects at cost agreed in the quote.
- According to projects’ milestones: UH transfers data in RDF or other agreed-on formats via BioMedIT to SPHN-funded projects at cost agreed in the quote.
Federated Query System (FQS):
- UH loads, at least monthly, consented data following agreed standards into their local instance of the FQS, according to the technical format specifications and quality guidelines from SPHN.
- UH provides first level IT support (through the application manager) and takes care of the technical maintenance (e.g., implementation of updates and new releases) for the Federated Query Tool.
Semantic-based data representation layer:
- UH maintains a person responsible for supporting semantic data representation within UH and in collaboration with SPHN projects as well as DCC.
- Within the yearly budget and Roadmap approved by UH and SPHN, the HIT-STAG has the mandate to define implementation of the SPHN Interoperability Framework.
- UH implements data standards (= specified and maintained/updated in the Clinical Data Warehouse (CDW) in the defined format OR: can be instantly delivered in the defined format) according to the separate HospFAIR agreement. The definition of key performance indicators and payment schedule is governed by the HospFAIR agreement.
- UH defines and enacts an internal policy on semantic management of data that enforces SPHN standards.
- UH defines and enacts an internal policy on semantic management of data that enforces SPHN standards.
Omics data and biosample interoperability:
- UH is technically able to provide a minimal set of biosample meta-data according to the Swiss Biobanking Platform (SBP) specifications for patient data under General Consent.
- UH is technically able to link sample information to clinical information in the CDW (ID management).






