Development of a governance and quality management system for exchange of patient related data for research purposes
Project consortium: Dr. Joerg Willers (University Basel/University Hospital Basel), Dr. Thomas Fabbro (University Basel/University Hospital Basel), Prof. Christiane Pauli-Magnus (University Basel/University Hospital Basel), Dr. Michael Weisskopf (University Hospital Zurich), Cornelia Kruschel-Weber (University Hospital Zurich), Prof. Gabriela Senti (University Hospital Zurich), Dr. Verena Pfeiffer (University of Bern), Annette Magnin (SCTO).
Acknowledgements: Prof. Alexander Leichtle, Dr. Dominique Furrer (Insel Data Coordination Lab & Science Center, Bern)
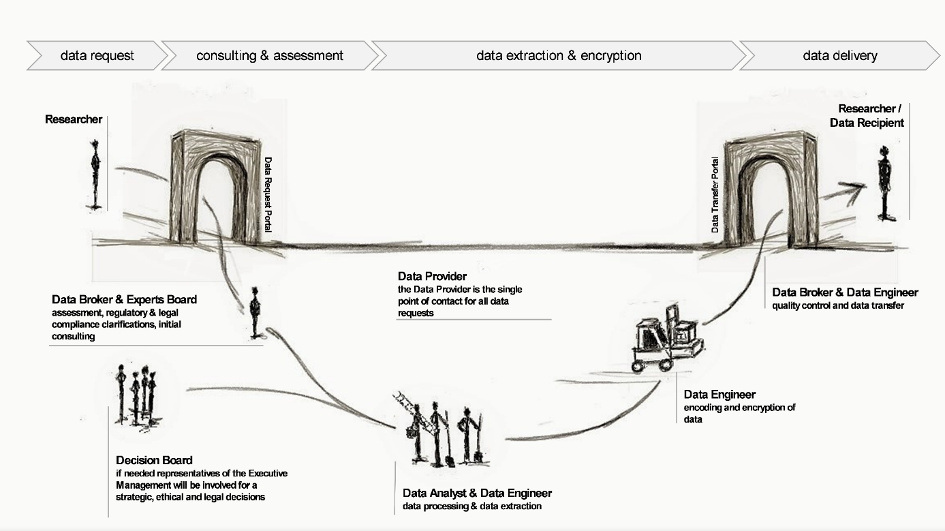
Figure 1 Data request and delivery work flow including roles & responsibilities for research purposes
Main achievements
The increasing demand for more, high quality and clinically annotated data repositories is without question. On a local (hospital) level or even on a department level activities are ongoing to generate such infrastructures. Providing a solid quality management system framework will allow the institution to act in compliance with applicable regulations and to provide biggest possible security for the patient data as large shared medical datasets provide major opportunities for improving health systems as well as individual care.
A set of documents has been generated (guidelines, processes, templates) that describe the proposed processes and considerations to standardize data request and data delivery processes for research purposes and, by this, to enable data providers (e.g. university hospitals) to ensure compliance with regulatory and internal requirements and high data quality (Figure 1). The key focus areas include data availability, usability, consistency, integrity, security, and furthermore, establishing processes to ensure effective data management throughout the whole data life cycle within a research project.
Reusable infrastructure and datasets
Data Governance Framework Summary Report
The above described process has been implemented in principle at the University Hospital Basel.
Phases of the process are determined in local Standard Operation Procedures (SOPs) and followed according to Figure 2.
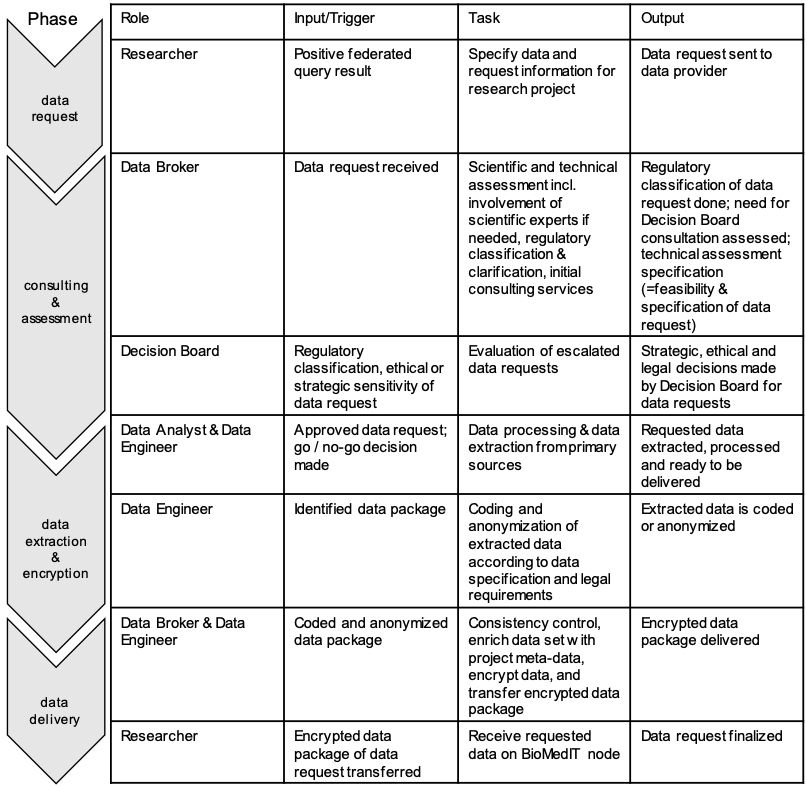
Figure 2 Description of tasks involved in a data request and the appropriate workflow
The three core elements or questions that have to be answered before the data can leave the providing institution are:
- Are the data consented? (i.e. is patient approval for use available?)
- Is an ethics approval necessary/available for further use?
- Is a contractual agreement between data provider and data receiver in place?
Available ressources
The developed tool can be accessed through the project development team or SPHN directly.
Follow-up projects
Any institution using clinical (patient) data for research and willing to join the SPHN network can make use of the developed data governance guidance documents and tools. Once endorsed or implemented, the institutions will develop an understanding of legal and ethical requirements allowing them to perform clinical research with sensitive data in a compliant manner.
Watch the SPHN webinar
The concept of this project is to develop and implement tools for data governance at an institutional level (e.g. rules and regulations, document templates) that allow a controlled and secure handling of sensitive data. By means of co-development, each project partner verifies the governance and quality management structure for its viability and generalizability. This should lead to a generic set of documents, suitable as templates for other hospitals or institutions that plan similar data sharing platforms.
Disclaimer: The contents on this website are intended as a general source of information and have been provided by the project PIs. The SPHN Management Office is not responsible for its accuracy, validity, or completeness.

